Some Facts About Helium
Contents
Discovery of Helium
Helium was first detected as an unknown, yellow spectral line signature in sunlight during the solar eclipse of 18 August 1868 by Georges Rayet, C. T. Haig, Norman R. Pogson, and John Herschel. It was confirmed by French astronomer Jules Janssen and the English astronomer Joseph Norman Lockyer. While Janssen observed the solar eclipse of 18 August 1868 at Guntur (Andhra Pradesh), Norman Lockyer observed it from Britain. Janssen noticed bright lines in the spectrum of the chromosphere, showing that the chromosphere is gaseous. However, only Lockyer proposed that the new yellow line near the sodium D-line, which he called “D3”, was due to a new chemical element, which he named after the Sun (from Greek: ἥλιος, helios).
On 26 March 1895, the Scottish chemist Sir William Ramsay isolated helium for the first time on Earth from the mineral cleveite (a variety of uraninite with at least 10% rare-earth elements). In the spectrum of the gas, which he liberated from the mineral by sulfuric acid, he noticed a bright yellow line, matching the D3 line observed in the spectrum of the Sun. These samples were identified as helium by Norman Lockyer and British physicist William Crookes. In the same year, helium was independently isolated from cleveite by Per Teodor Cleve and Abraham Langlet in Uppsala, Sweden, who collected enough of the gas to accurately determine its atomic weight.
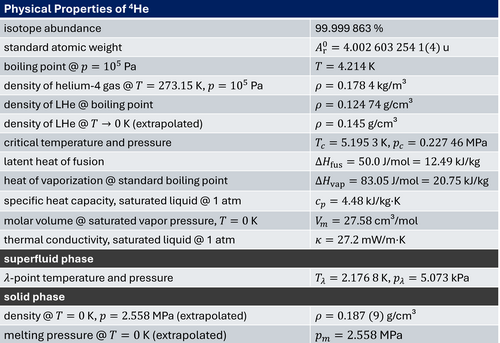
Physical Properties of Helium
The chemical element helium is the second-lightest chemical element. At ambient pressure, it is an inert, monatomic noble gas, which is colorless, odorless, and non-toxic. Its boiling point of 4.2 K is the lowest among all the chemical elements.
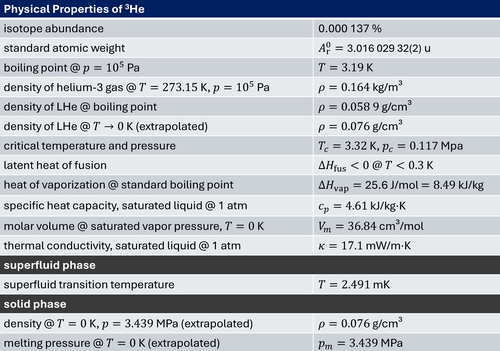
There are nine known isotopes of helium. However, only two of them, helium-3 and helium-4, are stable. In the Earth's atmosphere, the ratio of 3He and 4He atoms is about one over a million. Unlike most elements, helium's isotopic abundance varies greatly by origin, due to the different formation processes. Whereas 4He is produced on Earth by alpha decay of heavier radioactive elements, 3He is present on Earth only in trace amounts, with most of it being present since Earth's formation (although some may fall to Earth trapped in cosmic dust).
In 1908, helium was first liquefied by the Dutch physicist Heike Kamerlingh Onnes. He also tried to solidify it by further reducing the temperature. He failed, as helium does not freeze at ambient pressure, even at absolute zero temperature. Only in 1926, Onnes' student Willem Hendrik Keesom succeeded in solidifying helium by applying additional external pressure.
Superfluidity
In 1938, the Russian physicist Pyotr Leonidovich Kapitsa discovered that 4He has almost no viscosity at temperatures near absolute zero. This phenomenon, which is related to Bose–Einstein condensation of interacting bosons, is called superfluidity. In 1972, the same phenomenon was observed in 3He, but at temperatures much closer to absolute zero, by American physicists Douglas D. Osheroff, David M. Lee, and Robert C. Richardson. Since 3He is a fermion, whereas 4He is a boson, the phenomenon in 3He is related to the pairing of fermions, in analogy to the pairing of electrons to Cooper pairs in superconducting metals. Although 4He and 3He have the same electron configuration (1s2 ) with total electron spin S=0, the missing neutron in the 3He nucleus results in a different total nuclear spin (I=0 for 4He and I=1/2 for 3He). The resulting different quantum statistics (bosonic versus fermionic) results in a completely different low temperature behavior of liquid 4He and 3He (see e.g. R. Gross, Lecture Notes on Low Temperature Physics).
Where Does Helium Come From?
In general, helium is not a rare element in our universe. On the contrary, it is the second-most abundant element in the observable universe, after hydrogen. It represents about 24% of the total elemental mass, more than 12 times the mass of all the heavier elements combined. The most common isotope of helium in the universe is helium-4, which was formed during Big Bang nucleosynthesis. The nuclear fusion of hydrogen in stars permanently creates large amounts of new helium.
On Earth, however, helium is a rare element. The present helium has mostly been generated by the natural radioactive decay of heavy radioactive elements in the Earth’s crust, such as thorium (Th234) or uranium (U238). The alpha particles emitted by such decay consist of 4He nuclei. The helium generated by radioactive decay is contained in natural gas in concentrations as high as 7% by volume, from which it is extracted commercially by a low-temperature separation process called fractional distillation.
It is important to note that helium is an irreplaceable natural resource. Today, we extract it from natural gas, which is heavily mined to serve the energy market. For commercial reasons, only a few selected natural gas sources, which contain sufficiently large helium concentrations at a level of about 1%, are used to produce helium. Helium from natural gas sources with lower helium content is wasted into the atmosphere, although concentrations of less than 0.1% are viable if liquid natural gas extraction is used. As helium on Earth originates from alpha particles emitted by uranium and thorium isotopes, with long-lived radioactive decay taking hundreds of millions of years, it is quasi-non-renewable. Once released from the ground as a mixture with natural gas, it enters the atmosphere on much shorter time scales. Unfortunately, due to helium’s small particle size and mass, it is not gravitationally bound to Earth like other noble gases, and, finally, it will escape into space and be lost forever. Consequently, helium leakage presents a significant challenge in storage, transportation, and handling. Therefore, long-term helium supply is thought to be critical. However, we note that some studies suggest that helium produced deep in the Earth by radioactive decay is collected in natural gas reserves in larger-than-expected quantities.
As helium is an irreplaceable natural resource on short time scales, we should avoid wasting it. A seemingly egregious example of waste might appear to be party balloons. However, regarding the question of helium conservation, its specific end-use is irrelevant. Once helium is mined, refined, and distributed to users, there is just a short period until permanent loss. The only way to conserve helium over long timescales is to leave the helium-containing natural gas in the ground or, after extraction, store the helium in a tight reservoir. Until today, the only large storage facility was the US National Helium Reserve, which once held over 1 billion cubic meters (about 170,000,000 kg) of helium as a strategic reserve of the United States. Unfortunately, with the Helium Privatization Act of 1996, the US Congress has mandated that helium be sold off. In 2024, the remaining reserve was sold to the Messer Group. Consequently, today, there are no obvious conservation options.
Helium-4 Resources and their Accessibility
Helium-4 is considered a critical raw material (CRM) and, so far, has often been overlooked in the resource sustainability literature compared to metals and other resources. The exceptional physical and chemical properties of helium make it vital in diverse applications in healthcare, electronics, aerospace, military, and communications. In liquid form, helium is indispensable as a cryogenic coolant in applications such as MRI. Helium is also considered irreplaceable for research in physics, materials science, and energy-related technologies. Therefore, most industrial nations meanwhile classify helium as a “critical” resource. As nations perceive the importance of resource availability based on their industrial and technological needs, they may identify dependency on foreign sources as a strategic vulnerability for their economy. Therefore, helium resources, trading networks, geopolitical supply risks, and the accessibility of helium resources must be analyzed continuously.
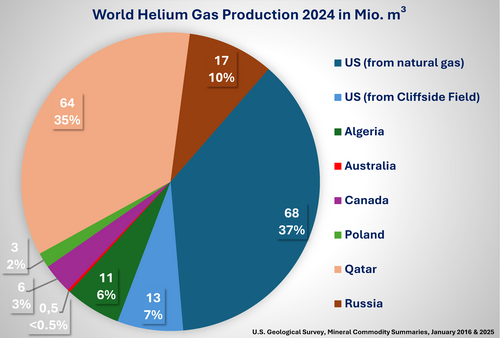
Since about 2015, Qatar has provided a stable increase in helium production, while exports from the other two leading producers, the United States and Algeria, have decreased. Smaller producers like Australia, Ukraine, Poland, and Russia have only contributed marginally to the overall supply. Still, they may become crucial as some of these suppliers are geographically proximate to major user countries like Germany, Japan (the most significant net importer in 2015), and China, which meanwhile overtook Japan as the biggest net importer.
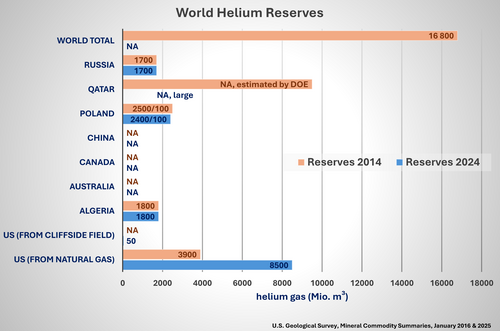
World Helium Reserves
The estimates of the world’s helium reserves have a large error bar. Since helium is contained in natural gas, the main resources are located in countries with the main resources of natural gas: the US, Qatar, Algeria, and Russia. The annual world helium production currently amounts to about 1% of the estimated helium reserves. Since new natural gas sources are expected to be discovered, the helium supply is likely to be safe for the next 100 years, even if the consumption is still increasing. However, one must remember that helium, once used in various applications, completely disappears into space and therefore cannot be recycled, like metals.
What Do We Use Helium-4 For?
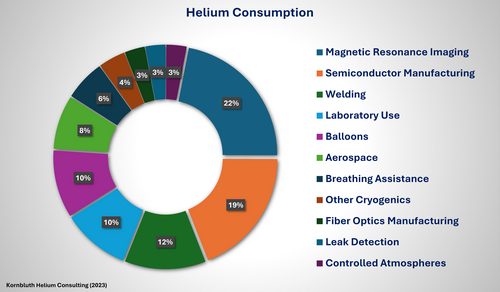
Today, more than one-third of the helium consumption is for cryogenic applications (mostly MRI: 22%, laboratory use: 10%, other cryogenics: 4%). Complementary use for non-cryogenic applications depends on other physical properties of helium: lifting power, chemical inertness, and purity, which are important in various manufacturing processes such as optical fiber production, the semiconductor industry, welding, and purging. Helium is particularly indispensable for low-temperature physics, chemistry, and life sciences experiments. However, usage for science presently accounts for just 10% of the total helium consumption worldwide, well below medical MRI (22%), semiconductor manufacturing (19%), welding cover gas (12%), or lifting gas in balloons (10%). Examples for helium usage are:
- Cooling down magnets in MRI machines that doctors use to examine people for cancer and other diseases. It is also used to cool down magnets in accelerators, fusion reactors, and in super-fast train systems such as the Shinkansen train in Japan.
- Deep-sea diving, requiring a mixture of 80% helium-20% oxygen.
- Border surveillance by helium-filled blimps.
- Cardio-pulmonary resuscitation pumps for heart surgery.
- Rare document preservation (i.e., US Declaration of Independence).
- Leak detection agent for extremely small leaks since helium does not react with other gases.
- Helium-Neon lasers for eye surgery.
In research, scientists and engineers from a wide spectrum of disciplines use liquid helium as a cooling liquid. In biology and chemistry, liquid helium is mostly used for high-resolution superconducting magnets for NMR, mass spectrometry, and other scientific instruments, while in medicine radiologists need liquid helium for their MRI magnets. In physics and material science, liquid helium is used in various facilities and for a plethora of experimental techniques. They range from large particle accelerator facilities in fundamental physics research, over smaller lab facilities used to study quantum materials and new physical phenomena, to device fabrication and characterization in metrology and quantum information technology.
In fundamental and applied research, addiction to helium is particularly strong among physicists. They need it as a coolant liquid to operate stable superconducting magnets that generate high magnetic fields, and to perform experiments at sub-kelvin temperatures. Fortunately, most large-scale facilities, which require large amounts of liquid helium, are equipped with efficient helium recovery systems. For example, the helium recovery system at SLAC’s Linac Coherent Light Source-II is among the largest cryoplants in the US, circulating 32,000 liters of LHe at 4.5 K to cool the superconducting RF cavities to 2 K. The Large Hadron Collider at CERN circulates a helium inventory of over 750,000 liters of LHe.
In physics, the dependency on liquid helium is directly related to its versatility as a cryogenic liquid. It does not freeze at ambient pressure, even at absolute zero temperature. It has large cooling power and does not cause significant vibrations. However, over the past decades, helium has become less available and therefore more expensive. Thus, solutions – so-called cryogen-free cooling techniques – have been developed to replace liquid helium. In science, we meanwhile use various closed-cycle cooling machines such as pulse-tube refrigerators and cooling techniques based on electron and nuclear demagnetization techniques. However, cryogen-free systems cannot completely eliminate the need for liquid helium. Today, so-called dry fridges are already widely used in certain applications, such as in quantum information science, where the vibrations they create are not an issue. However, this is not true for other low-temperature applications, such as scanning tunneling microscopy, where dry fridges may not be usable, even if they will be able to better mitigate vibration. Magnetic cooling techniques are disadvantageous for applications requiring very low magnetic stray fields, such as superconducting quantum computing.
The History and Status of Helium Production
On Earth, helium is relatively rare (5.2 ppm by volume in the atmosphere) and, therefore, direct extraction from the atmosphere is too expensive. In 1903, an oil drilling operation in Kansas produced a gas geyser that did not burn. Analyzing the gas, it was discovered that 1.84% of the gas sample was helium. This showed that despite its overall rarity on Earth, helium was concentrated in large quantities under the American Great Plains, and that it can be extracted as a byproduct of natural gas.
Production from helium-rich gas wells in the regions of Texas, Oklahoma, and Kansas started in 1920 with the Mineral Leasing Act, motivated by military interest in its lifting power. A total of 5,700 m3 of 92% helium was produced, although less than a cubic meter of the gas had previously been obtained. Although the helium extraction by low-temperature gas liquefaction was not yet developed during World War I, helium production continued, mainly for use as a non-flammable lifting gas in lighter-than-air craft. During World War II, the demand for helium increased. Besides using it as a lifting gas, it was important for shielded arc welding and the helium mass spectrometer, which was vital in the atomic bomb Manhattan Project. Helium produced between 1930 and 1945 was about 98.3% pure (2% nitrogen), which was adequate for airships. In 1945, a small amount of 99.9% helium was produced for welding use, and by 1949, commercial quantities of Grade A 99.95% helium were available
Already in 1925, the US government decided to set up a National Helium Reserve at Amarillo, Texas, managed by the Bureau of Land Management (BLM) of the United States. The goal was to extract helium gas from what is known as the Bush Dome Reservoir and supply military airships in time of war and commercial airships in peacetime. With the Helium Act of 1925, the US banned the export of scarce helium. Consequently, German Zeppelins were forced to use hydrogen as lifting gas, which then gained infamy in the Hindenburg disaster. After World War II, the helium market was depressed, but the reserve was expanded in the 1950s to ensure a supply of liquid helium as a coolant to create oxygen/hydrogen rocket fuel (among other uses) during the Space Race and Cold War. Helium use in the United States in 1965 was more than eight times the peak wartime consumption.
After the Helium Act Amendments of 1960 (Public Law 86–777), the U.S. Bureau of Mines arranged for five private plants to recover helium from natural gas. For strategic reasons, the US decided to purchase crude helium from these private producers and store it. For this purpose, the Bureau built a several 100 km long helium pipeline system running through Kansas, Oklahoma, and Texas (the Helium Pipeline) that connects crude helium extraction plants with each other, with helium refining facilities, and with the Bush Dome Reservoir, a naturally occurring underground structural dome near Amarillo, Texas. There, crude helium was stored and purified later when it was needed. However, there was the caveat that the government should be paid back by 1995. This halted accumulation of helium after the mid-1980s, and there has been no net transfer into the National Reserve since that time. The Reserve was rather used as a buffer to compensate for fluctuations in production and sales.
By 1995, a billion cubic meters of the gas had been collected – about six times the annual world production today. Furthermore, at that time, principal and interest owed to the US Treasury were 1.3 billion dollars, prompting the Congress of the United States in 1996 to discontinue the reserve. In 1996, the Helium Privatization Act (HPA) was passed, requiring complete liquidation of the helium stocks down to a level of 2,000 cubic meters by a new deadline of January 2015, or repayment of debt, whichever came first. The fact that BLM had to exit the helium business had severe consequences on the helium market. The customers faced the problem of an abrupt loss of the helium supply from the US Helium Reserve, leading to a decrease of 30% in the world supply. Fortunately, the US Congress stepped forward with yet another action, the Helium Stewardship Act (HSA) in 2013. This act extended the operation of the Helium Reserve to autumn 2021, allowing it to feed the still-growing helium market, mostly driven by manufacturing interests. The HSA also stated that the Helium Reserve facilities would be finally “designated as excess property and be disposed of” in 2021.
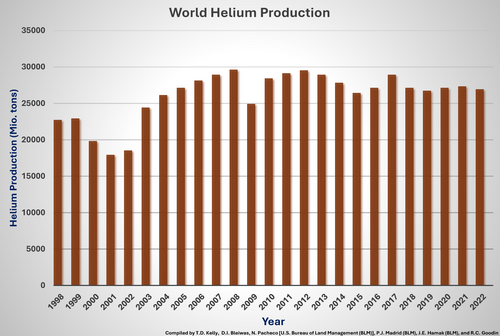
For many years, the United States produced more than 90% of commercially usable helium in the world. The rest was delivered by extraction plants in Canada, Poland, Russia, and other nations. Today, the annual global helium production amounts to about 160 to 180 million cubic meters (27,000 to 32,000 tons). It is produced mostly in the USA, Qatar, Algeria, and Russia, and then mainly transported as a cryogenic liquid to the customers.
The Helium World Market Price
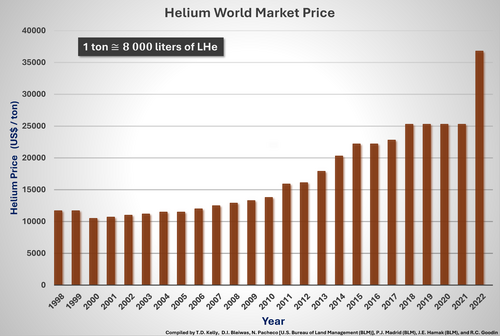
Over the past two decades, the helium price has been strongly increasing. The reason is a growing demand and a change in supply chains. Although helium prices were doubling from 2002 to 2012, both helium consumption and the costs of producing helium increased further. There are several reasons for the continuously increasing helium prices. As of 2012, the US National Reserve still accounted for about 30% of the world's helium. However, its National Reserve was running out of helium in 2021. Although other large reserves are around (e.g., in the Hugoton in Kansas, and nearby gas fields of Kansas and the panhandles of Texas and Oklahoma) and new helium plants opened in Qatar, Russia, and the US state of Wyoming, a general shortage in helium has developed, keeping the price at a high level. Therefore, helium recovery systems become more attractive, and customers have to develop techniques that no longer require helium, such as helium-free cooling systems.
Regarding new sources of helium production, in the mid-1990s, a new plant in Arzew, Algeria, began operation with enough production (about 17 million cubic meters) to cover Europe's demand. Furthermore, in 2004–2006, additional plants in Ras Laffan, Qatar, and Skikda, Algeria, were built, advancing Algeria quickly to the second leading producer of helium. However, the facilities in Algeria usually produce helium as a result of the liquefaction of natural gas, which is transported to the customers via container ships. Only this liquefaction process of natural gas makes the production of helium economically viable at these facilities. Due to the war in Ukraine, European natural gas supplies are currently highly restricted and most of the natural gas from Algeria is being transported directly by pipeline to Europe via Spain. As transport through a pipeline does not require liquefaction, the extraction of helium is currently not economically viable, leading to a reduction in global supply.
Moreover, a new production plant in the Amur region of Russia was expected to add a further 20 to 30% capacity (up to 70 million cubic meters) into global helium supply by 2023. However, a fire in October 2021 followed by an explosion and fire in January 2022 has resulted in zero capacity from this plant. At present, it is unknown when this plant will be in operation again. Moreover, Russia’s war with Ukraine has led to an embargo of oil and gas product imports.
These are just some of the reasons for problems in the current helium supply chain and the related increase of helium price. Other factors include an embargo of gas from Qatar passing through Saudi Arabia to a large port in the United Arab Emirates. Although this problem has meanwhile been resolved, there are still various maintenance and unplanned outages from a range of smaller production facilities globally. Altogether, this results in a challenging situation in helium supply.
Looking ahead, helium markets and pricing are expected to become more complicated than in the past. The main reason is that the US no longer balances out fluctuations in helium production by its National Reserve. This encourages more private sector involvement in international production and markets, but also is expected to lead to larger price fluctuations and supply risks. Managing supply risks, e.g., by entering long-term supply contracts with multiple stable suppliers, will become increasingly important. However, there is hope that the helium supply situation improves in the medium-to long-term. First, a large gas supplier recently announced the opening of the world’s largest helium storage cavern facility in Texas, with a supply capacity that exceeds that of the BLM plant. Secondly, new companies are forming that aim to extract helium as an important resource. That is, these companies aim at mining and exploring helium gas rather than considering helium production as a side business of natural gas refining. New sites are being developed in the U.S. in Saskatchewan and Utah, as well as in Tanzania, and may contribute a significant capacity in the near future.
Literature
- D. Kramer, Helium prices surge to record levels as shortage continues, Physics Today 76 (9), 18-20 (2023).
- W.P. Halperin, The impact of helium shortages on basic research, Nature Physics 10, 467-470 (2014).
- National Research Council. 2010. Selling the Nation's Helium Reserve. Washington, DC: The National Academies Press.
- A. Siddhantakar et al., Helium resource global supply and demand-Geopolitical supply risk analysis, Resources, Conservation & Recycling 193,106935 (2023).
- Randell F. Barron, Cryogenic Systems, Oxford University Press (1985), ISBN: 0195035674, 9780195035674.
- Frank Pobell, Matter and Methods at Low Temperatures, Springer-Verlag (1992), https://doi.org/10.1007/978-3-540-46360-3.
- R. Gross, Superconductivity and Low Temperature Physics 2, Walther-Meißner-Institute (2004-2024).